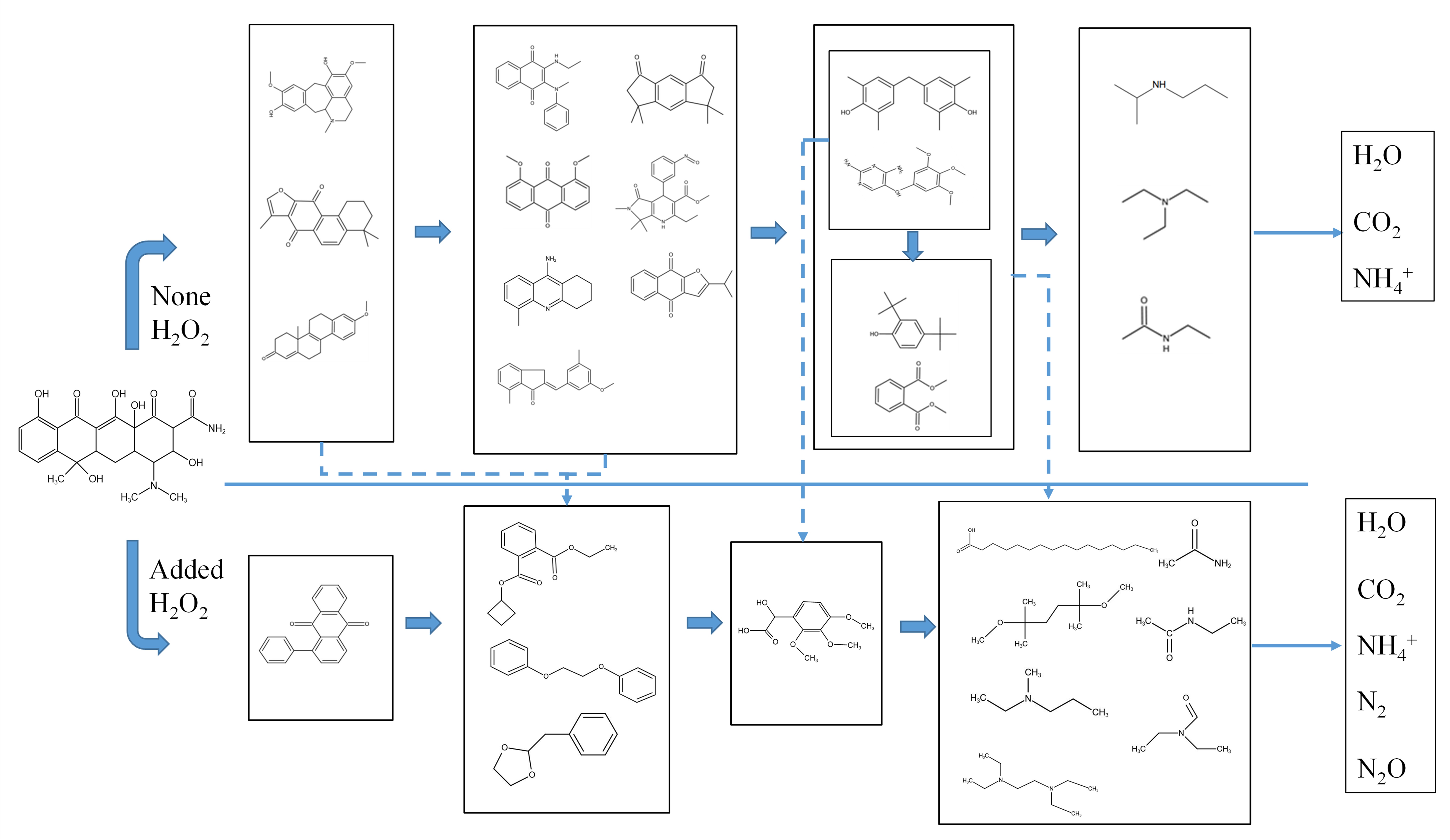
Degradation of tetracycline hydrochloride in sub- and supercritical water with and without oxidation
- DOI码:10.1016/j.psep.2022.04.030
- 发表刊物:Process Safety and Environmental Protection
- 摘要:In the present work, in a batch quartz tube reactor, supercritical water gasification and supercritical water oxidation were used to treat tetracycline hydrochloride (TC). The results show that the increase in temperature, the extension of reaction time, and the decrease in initial concentration all had a positive impact on the removal of TC. After adding 0.108 mol/L H2O2 (n = 1), the removal rate reached 100% at 1000 mg/L, 400 °C and 100 s. First-order degradation kinetics was used to fit the degradation of TC. In the absence of H2O2, its pre-exponential factor and activation energy were found to be 0.03 s−1 and 6.14 kJ/mol, respectively. After adding H2O2, its pre-exponential factor and activation energy were found to be 6.70 s−1 and 25.66 kJ/mol, respectively. The degradation process of TC was explored and the results show that TC can be degraded into smaller molecular substances in supercritical water. After adding H2O2, the degradation efficiency of TC was significantly improved the degradation process was also more thorough. There were two main degradation processes, namely the ring-opening reaction and the central carbon cracking.
- 卷号:162
- 期号:0
- 页面范围:373-383
- 是否译文:否
- 发表时间:2022-04-13
- 收录刊物:SCI
- 发布期刊链接:https://www.sciencedirect.com/science/article/pii/S0957582022003354
附件:
final.pdf