Lewis acid catalyzed gasification of humic acid in supercritical water
- DOI码:10.1016/j.cattod.2017.02.017
- 发表刊物:Catalysis Today
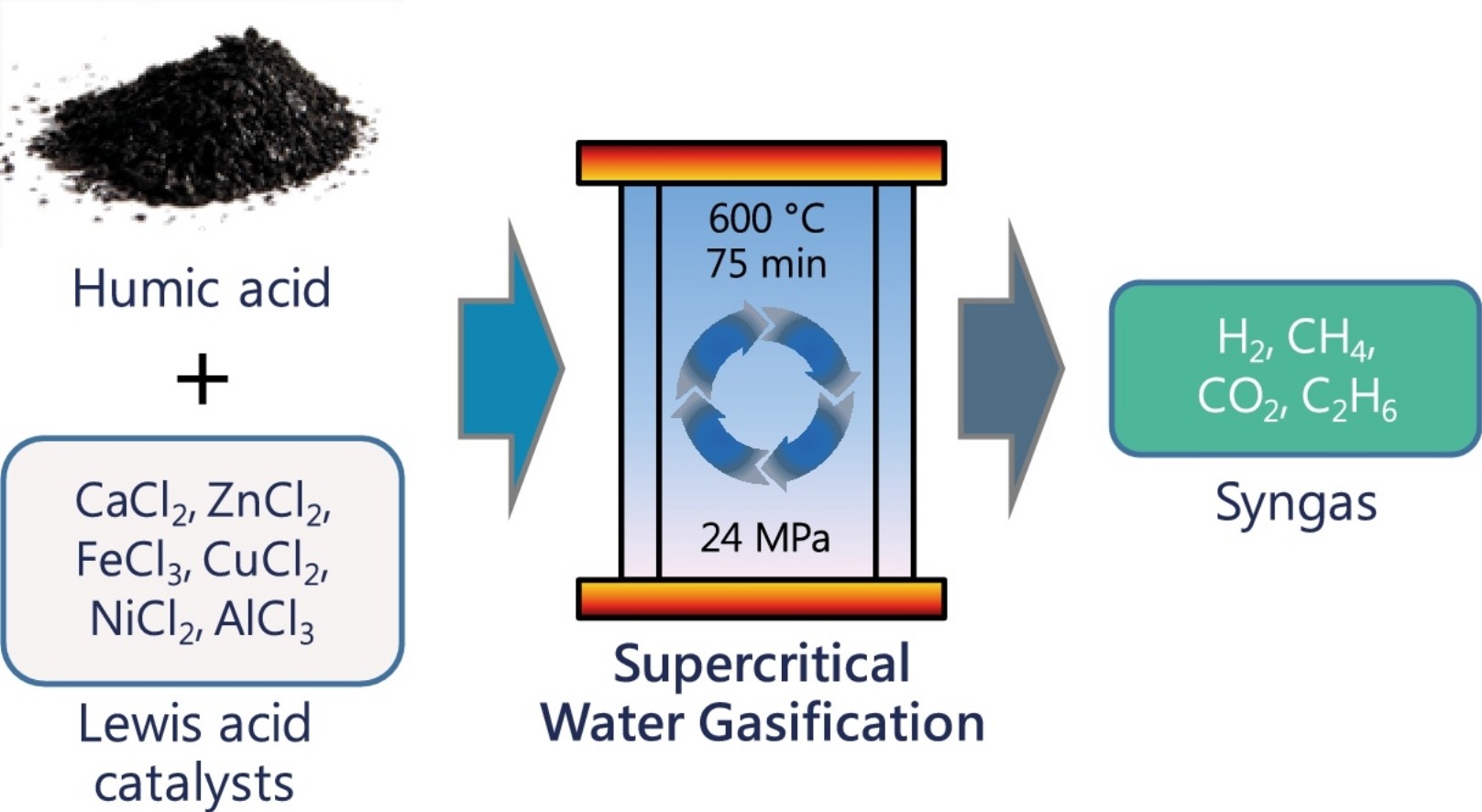
- 摘要:Humic acid is a model compound of sewage sludge that occurs as a result of decomposing organic matter. Supercritical water gasification of humic acid was performed at a temperature, feed concentration and reaction time of 600 degrees C, 15 wt% and 75 min, respectively with variable concentrations (5-15 wt%) of Lewis acid metal chloride catalysts. The non-catalytic gasification of humic acid resulted in H-2 yield (0.79 mol/kg), total gas yield (1.59 mol/kg), carbon gasification efficiency (2.2%), H-2 gasification efficiency (10%) and lower heating value of 5.1 kJ/Nm(3). In contrast, 15 wt% of Lewis acid catalysts loading significantly improved H-2 yields (2.79-11.03 mol/kg), total gas yields (4.72-15.44 mol/kg), carbon gasification efficiency (5.7-12.4%), H-2 gasification efficiency (40.9-141.6%), energy recovery (8.8-33.79%) and lower heating value of gas products (22.4-72.3 kl/Nm(3)). The activity of Lewis acids was in the order: CaCl2 < ZnCl2 < FeCl3 < CuCl2 < NiCl2 < AlCl3. The addition of Lewis acid catalysts led to an increment in H-2 yield due to ring-opening reaction and intermediates gasification. High catalyst loading promoted the degradation of humic acid with greater gas yields and fragmented surface morphology of char residues. The liquid effluents from Lewis acid catalyzed gasification of humic acid contained considerable amounts of aromatic and aliphatic components.
- 卷号:291
- 期号:0
- 页面范围:13-23
- 是否译文:否
- 发表时间:2017-03-22
- 收录刊物:SCI
- 发布期刊链接:https://www.sciencedirect.com/science/article/abs/pii/S0920586117300792
附件:
Gong-2017-Lewis acid catalyzed.pdf